In this article, we will find out if CH2Br2 is polar or not. So, is CH2Br2 polar or nonpolar? CH2Br2 (dibromomethane) is a polar molecule due to the large difference between the electronegativity of H (2.2) and Br (2.96) atoms that gain partial positive and negative charge respectively. The net dipole moment of CH2Br2 is 1.7 D making it a polar molecule.
Why is CH2Br2 Polar?
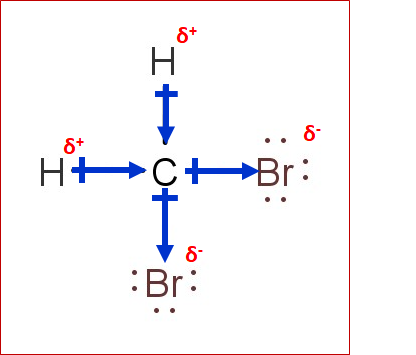
The answer can be illustrated by looking at its Lewis structure. The tetravalent carbon atom is singly bonded to two H-atoms and two Br-atoms. Even though the structure is symmetrical, their electronegativities are vastly different. The two bromines and two hydrogens are not opposed to each other, resulting in a difference in electronegativity. This is how CH2Br2 becomes polar. Here is the Lewis structure of CH2Br2:
What are the Lewis Structures?
These are the dot structures of compounds that visualize the number of valence electrons in a molecule. These structures help in determining the overall polarity of the compound.
Steps to find the Polarity of Molecule
whether a molecule is polar or not when electronegativity is not specified? The answer is yes. Here are some instructions to guide you:
- Draw Lewis Structure.
- With the application of VSEPR theory, find out the geometry of the molecule.
- Look at the resultant dipole moment.
- When the resultant dipole moment is 0, it is non-polar. If non-zero, it is polar.
What is Polarity?
Anything with a charge has the potential to be polar. Polarity, also known as the dipole moment (μ=Qr) in a stricter meaning, is the spatial difference of charges. In the case of polarity, there are two types of studies: bond polarity and molecule polarity.
What is Bond Polarity?
Uneven distribution of charges across a bond is generally due to electronegativity difference. Electrons are strongly held by the atom that has a higher electronegativity. In the process atom with a higher electron affinity acquires a partially negative charge. An atom having lesser electron affinity acquires a partially positive charge. The difference in electronegativity should be above 0.5 for the bond to be polar. To find the bond polarity, one has to simply look at the electronegativity values of atoms in the bond. Increased polarity dictates the molecule to act more ionic, whereas decreased polarity makes the bond more covalent. Test Your Application Try to answer this question. Which bond is the most polar?
Cl−FCl−F Br−FBr−F I−FI−F F−F
Answer: The options include halogens. The order of electronegativity of halogens is F > Cl > Br > I. Because fluorine and iodine are the highest and least electronegative elements, respectively, their bonds will be highly polarized. The I-F bond is the most polar.
What is Molecular Polarity?
Like bond polarity, it means the uneven distribution of charges in a molecule. Some atoms that have greater electron affinity attract the electron towards them and gain a partial negative charge. Atoms that have less electron affinity acquire a partial positive charge as a result. The procedure of identifying whether or not a molecule is polar is more complex. Compound structures are also considered when determining whether a molecule is polar or not. In this scenario, a solid understanding of Lewis structures and VSEPR theory is essential.
VSEPR Theory
Sidgwick and Powell presented the Valence Shell Electron Repulsion Theory in 1940. This is a model used in chemistry to predict the overall geometry of a molecule. This model assumes that the resultant structure has a minimum repulsion among its electron pairs.
VSEPR Theory Postulates
• When a molecule consists of more than three atoms, a central atom is chosen. This is the atom to which other atoms are bonded. • The shape of the molecule is governed by the number of valence shell electron pairs. • Due to repulsion between electron pairs, the molecule tends to take a shape with an orientation where repulsion felt by each atom is minimized. • The valence shell is a spherical space where the electrons are localized on the surface in a way that has the maximum distance between them. • An asymmetrical shape is observed when the central atom has bond pair electrons surrounding itself. • A distorted shape is expected in a case where the central atom has both bond pairs and lone pairs surrounding itself. • The VSEPR theory is applicable to resonance structures of molecules as well. ∙ The order of strength of repulsion among electron pairs is as follows: Lone pair – Lone pair > Lone pair – Bond pair > Bond pair – Bond pair • The increased energy of molecules is a result of the minimized distance between electron pairs. Shorter distances result in greater repulsion among electron pairs. • When the electrons are farther apart from one another, the repulsion felt by them is diminished. Thus, the energy of the molecule is also low.
Predicting the Shapes of Molecules
The following steps must be followed in order to decide the shape of a molecule. • The central atom is the one that has the least electron affinity. This is because these atoms are able to share electrons more efficiently with others. • Count the number of valence electrons in the central atom and add one electron for each bonding atom. • Look at the charge on the central metal atom; if the central metal atom is positive, subtract 1 electron. If the charge is negative, add 1 electron. • The total number of electron pairs is obtained on the division of these two numbers by two. Here in CH2Br2, Valence electrons = 4 Bonding electrons from 4 surrounding atoms = 4*1 VSEPR Number = (4 + 4)/2= 4 ie; Tetrahedral This number helps in the prediction of shape. (Refer to the below table)
VSEP number is helpful in predicting the overall shape of the molecule. When the number is 2, the molecule is linear, when 3; it is trigonal planar so on. Test Your Application
BrF5
Geometry: Square Pyramidal Polarity: Polar BrF5 Lewis Structure, Geometry, Hybridization
CCl4
Geometry: Tetrahedral Polarity: Non-polar CCl4 Lewis Structure, Geometry, Hybridization
Factors that determine the polarity of CH2Br2
Electronegativity
The dipole moment is the measure of polarity in a molecule. From VSEPR postulates, we know as the distance of the dipole increases, polarity decreases. Similarly, polarity increases with a decrease in distance. The dipole moment of dibromomethane: 1.7 D
The structure above illustrates the polarity in the molecule. The central atom (carbon) is attached by two H and Br atoms that acquire partially positive and partially negative charges that do not cancel each other. The larger the difference in electronegativity of two atoms, the more polar the bond becomes.
Geometry
VSEPR theory also accounts for this factor in the polarity of dibromomethane. We learned that molecules orient themselves in such a way that has the least repulsion among their electron pairs. Here, the molecule in question has lone pairs on Bromine atoms and not on Carbon atoms. So, it acquires tetrahedral geometry
Bond angle: 109 degrees.
Despite having the same geometry, why are CCl4 non-polar and CH2Br2 polar?
There are four Cl atoms attached around C-atom in CCl4 and thus, their electronegativities cancel out each other, resulting in a non-polar structure. In CH2Br2 however, the two Br and H atoms are not attached opposite to each other and thus, their electronegativities do not cancel out each other, resulting in a polar structure.
Does the Presence of lone pairs affect the dipole?
No, the dipole moment, the measure of polarity, is not directly influenced by the presence of lone pairs. However, lone pairs are special cases when determining the overall geometry of a molecule which consequently affects the dipole.
Conclusion
In this article, we attempted to solve your doubts regarding the concept of the polarity of CH2Br2. We illustrated a clear guide to finding polarity by taking the two specific case studies of polarity. We hope this succeeded in answering all your questions regarding the concept. The focus of our previous articles has been to educate on the fundamentals of polarity such as ‘what is polarity?’ and ‘factors affecting polarity’. So, determination of polarity was the key focus in this article today. This article also emphasized the study of Lewis dot structures and VSEPR theory in order to determine polarity in molecules.
Some key takeaways from this article
Polarity is the uneven distribution of spatial charge For the bond to be polar, the electronegative difference has to be 0.5 There are two polarities; bond polarity and molecular polarity To find bond polarity, only electronegativity difference is taken into account. Geometry and Lewis dot structures are taken into account to find molecular polarity, The dipole moment is a measure of polarity in the compound. The polarity of a molecule also depends on the length of the dipole. Dipoles that are opposite to each other cancel out one another.