Being diprotic it is responsible for the formation of two types of salts viz. hydrogen carbonates (HCO3-) and carbonates (CO3-2). Many of the students have questions about the acidity of H2CO3 (carbonic acid), whether it is a strong or a weak acid. So, In this article, we will study the acidity of H2CO3 and will let you know all the fundamentals. So, Is carbonic acid a strong acid? No, H2CO3 is not a strong acid as it does not dissociate completely in an aqueous solution. The carbonic acid molecule has two hydrogen atoms to lose i.e. it is a diprotic acid and, therefore, has two acid dissociation constants, Ka. The value of the acid dissociation constant is the reflection of the strength of an acid. The higher value of Ka indicates the higher strength of the acid. The reaction equations along with their Ka values are given below: H2CO3(aq) <=====> HCO3- + H+ Ka1 = 4.3 X 10−7 mol/L; pKa1 = 6.36 at 25°C HCO3- <=====> CO3-2 + H+ Ka2 = 4.8 X 10−11 mol/L; pKa2 = 10.25 at 25°C It is clear from the above equations that the value of the acid dissociation constant is very low while that of the logarithmic constant is higher for carbonic acid in comparison to strong acids. In the case of the strong acids, the Ka value is expected to reach infinity while the value of pKa should lie below 2. Hence, carbonic acid is not a strong acid. The pH of 0.1M carbonic acid is 3.68.
Why is Carbonic Acid a Weak Acid?
As discussed in the above section carbonic acid at first ionizes into bicarbonate and proton when it is dissolved in an aqueous solution. This means that the bicarbonate ion in this reaction works as the conjugate base for carbonic acid. Also, it is well known that bicarbonate ion is a strong conjugate base and therefore, has the tendency to remain associated with its proton due to which the acid molecules only ionize partially in the aqueous solution.
Which one is more acidic H2CO3 or CH3COOH?
Both the carbonic acid as well as acetic acid are placed under the category of weak acids and they do not ionize completely in an aqueous solution. However, the Ka value for carbonic acid is 4.3 X 10−7mol/L while that of acetic acid is 1.8 X 10-5 mol/L. Also, the pKa value of carbonic acid is 6.36 for the first dissociation equation while it is 4.76 for acetic acid.
These values indicate that acetic acid is stronger than carbonic acid. Now let us try to understand the reason for the greater strength of acetic acid in comparison to carbonic acid. Start with writing the dissociation equation for both the acids: For carbonic acid, H2CO3(aq) <====> HCO3- + H+ For acetic acid, CH3COOH(aq) <====> CH3COO- + H+ As per the above equations, the conjugate base for carbonic acid is bicarbonate ion while for acetic acid the acetate ion serves as the conjugate base. Check out the detailed article I wrote on is Acetic acid a strong acid. For now, we can leave the second dissociation equation of carbonic acid as the strength is measured only through the first proton released. Now, we have to analyze the strength of these conjugate bases as a stronger conjugate base means a weaker acid and vice versa. The acetate ion is a stronger conjugate base than the bicarbonate ion due to the resonance effect of the carboxyl ion. Another factor is the instability of carbonic acid which mostly decomposes to water and carbon dioxide molecules and a very little amount of carbonic acid is left in the solution due to which the acidity declines automatically. If you also want to know the chemical structure of Carbonic acid. read out the article on lewis structure of Carbonic acid.
The pH of Carbonic Acid
The pH scale is the one used for expressing the acidity or basicity of a substance in an aqueous solution. The values on the pH scale range between 1 and 14. All the acids have a pH value below 7 while all the bases have a pH value above 7. The neutral solutions such as pure water have a pH value of 7. Now, while calculating the pH value of carbonic acid we must keep in mind that it is a polyprotic acid i.e. it dissociates more than one time in a solution and thus releases different ions along with the release of protons. Hence, the steps for calculating the pH of 0.1 M carbonic acid will differ from other simple acids. First, let us start with the first dissociation equation resulting in the release of bicarbonate ions along with protons. The chemical equation is given below: H2CO3(aq) <====> HCO3- + H+ Now, calculate the hydrogen ion concentration of this equation knowing that the Ka1 value is 4.3 X 10−7 mol/L. The equation is written below: Ka = ([HCO3-] [H+]) / H2CO3 We are calculating pH for 0.1 M solution, therefore, the initial concentration of H2CO3 is taken as 0.1. Assuming that finally x protons and x bicarbonate ions are formed the above equation can be written as: 4.3 X 10^−7 = x^2/ 1-x x = 2.1 X 10^-4 Now, looking at the second dissociation equation the value of Ka2 is 4.8 X 10−11 mol/L. The equation is written as: HCO3- <====> CO3-2 + H+ Again, Ka2 = ([CO3-2] [H+]) / [HCO3-] As calculated in the above equation the concentration of HCO3- is 2.1 X 10-4. Now putting the values in the above equation assuming that y protons and y CO3-2 ions are formed, 4.8 X 10^−11 = (2.1 X 10^-4 + y)y / 2.1 X 10^-4 – y y = 4.8 X 10^−11 In the case of carbonic acid, the effect of the release of the second proton is completely negligible and the value of pH is derived from the first equation i.e. the value of Ka1. Therefore, pH = -log [H+] = -log[2.1 X 10^-4] = 3.68
What are Acids?
A substance that easily gives away protons when dissolved in water is known as an acid. These are the substances that are sour in taste, have pH below 7, turn blue litmus to red, and form a salt with alkalis. Based upon the extent of their dissociation in an aqueous solution acids are further classified as strong and weak acids. The acids that ionize completely are known as strong acids and those that do not dissociate completely in aqueous solutions are known as weak acids. Three theories are trusted to have correctly defined the properties of acids. Bronsted-Lowry Theory defines acids as the substance that gives away protons in an aqueous solution or is a proton donor. Arrhenius Theory states that acids produce hydrogen ions in water. Lewis Theory defines acids as electron acceptors. If a substance is said to have any or all of the above-listed properties it can be called an acid.
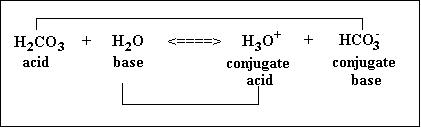
Conjugate Acid-Base Pairs
As per the Bronsted-Lowry theory of acids and bases, a substance that gives away its proton is an acid while the substance that accepts the protons is a base. For example, in the case of carbonic acid the dissociation equation is written as follows: H2CO3 + H2O <====> HCO3- (conjugate base) + H3O+ (conjugate acid) Here, H2CO3 gives away a proton and is, therefore, an acid while water accepts a proton and hence behaves as a base in this reaction. However, the theory further states that due to this loss and gain of a proton a conjugate base and conjugate acid are formed corresponding to the acid and base involved as reactants. In the above example HCO3- is the conjugate base of carbonic acid while H3O+ is the conjugate acid of the water molecule. A strong conjugate base indicates a weak acid and vice versa. Similarly, a strong conjugate acid indicates a weak base and vice versa. Therefore, these help in estimating the strength of acids and bases.
How is Carbonic Acid Formed?
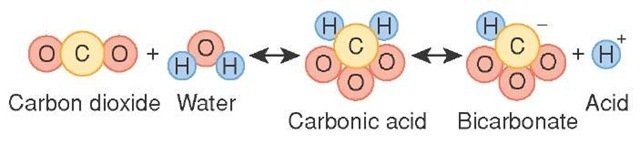
Carbonic acid is produced by dissolving carbon dioxide in water. The carbon dioxide molecules behave as an anhydride of carbonic acid and easily accept a water molecule to form carbonic acid. The reaction equation is written as follows: CO2 + H2O <====> H2CO3 It is unstable and weak and, therefore, easily dissociates into bicarbonate ions and hydrogen.
Is carbonic acid an organic or inorganic acid?
The compounds containing carbon and hydrogen atoms are referred to as organic compounds. There is a special branch of chemistry that deals with the properties and reactivity of these compounds. Although carbonic acid contains both carbon and hydrogen, it is still not considered an organic compound. Similar to carbon dioxide, carbonic acid is also an inorganic or mineral acid. The reason for this behavior can be understood by looking at the structure of the carbonic acid molecule which consists of one carbon-oxygen double bond and two carbon-oxygen single bonds.
Properties of Carbonic Acid
Carbonic acid is an unstable and weak dibasic or diprotic acid. It also occurs naturally inside the human body. A few important properties of carbonic acid are given in the table below:
Uses of Carbonic Acid
Some important uses of carbonic acid are listed below: • It is used in the formation of salts. • In our body the conjugate base of carbonic acid, bicarbonate ion is used in the transportation of CO2 outside through respiratory exchange. • In blood serum, also helps in the protonation of nitrogen bases. • It also acts as the buffering agent in our body and is broken into carbon dioxide by carbonic anhydrase activity. • It is used in the preparation of aerated drinks, sparkling wine, and carbonated water. • It also forms a part of mouthwash and vaginal washes. • Carbonic acid is used as a cleanser for contact lenses. • It is also used in the cosmetics and food processing industries. • It helps in the hydrolysis of starch.
Conclusion
Carbonic acid is a weak acid as it does not dissociate completely in the aqueous solution. The high value of the acid dissociation constant and the lower value of the logarithmic constant for carbonic acid further indicate that it is a weak acid. Amongst carbonic acid and acetic acid, H2CO3 is weaker due to the higher stability of the conjugate base of its conjugate base. Moreover, it has the tendency to reform its constituent compounds i.e. water and carbon dioxide. The conjugate base of carbonic acid is HCO3-. If you liked the article then share it in your groups. And feel free to suggest topics to write an article on. Happy learning!!